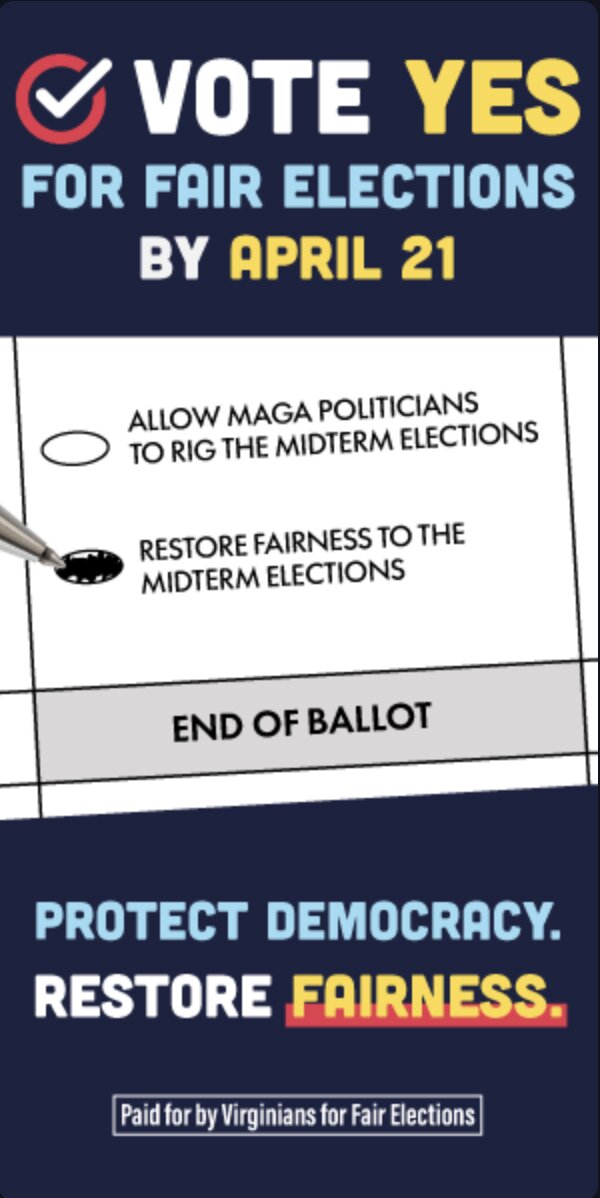
SPONSORED CONTENT
There are millions of women in the United States who have had breast implant surgery. The benefits of this surgery can be transformative, but there was a recent report by the FDA that says certain breast implants, specifically textured implants, may cause the non-Hodgkin’s lymphoma cancer strain, BIA-ALCL.
This report was released on July 24, 2019, and understandably millions of women across the country with breast implants are alarmed. Specifically, the report requested that Allergan voluntarily recall their textured breast implants without the Government forcing them to do so — and they complied.
In an official statement from the FDA, they said they took significant action to protect women in their recall in order to protect anyone who’s received breast implants that could be associated with anaplastic large cell lymphoma (BIA-ALCL). The maker of the textured implants in question is Allergan, which is why the FDA asked them to recall those specific type of implants.
Allergan complied with the request of their textured implant, which is their BIOCELL textured implant. There is no evidence that shows the additional tissue expanders, which can come with BIOCELL implants, may cause cancer.
The good news is that only 5% of the women in the US have textured implants, and since the recall is specifically for the macro-textured implant, this number is even lower. The implant recalled by Allergan is a macro-textured implant that has large, deep divots on the surface to help the implant stay in place. There are theories that this kind of implant may leave more plastic debris in the cavity than other implants, which is the cause of the cancer.
“This was a good move by the FDA to request a recall of Allergan’s BIOCELL implant. It shows that the Government is invested in protecting women’s health,” says personal injury attorney Tyson Mutrux of Mutrux Firm Injury Lawyers. “The first case of breast implant-associated cancer was reported in 1997 and the FDA first alerted the public to the danger in 2011. Cancer caused by breast implants is sadly nothing new. Thankfully more people are becoming aware.”
The FDA released the volunteer recall this Summer when 24 deaths associated with this type of cancer was made public. Although this number is extremely low, with only 33 deaths worldwide linked to the BIA-ALCL cancer strain, this number was enough for the FDA to do something and to no longer keep seeing cancer deaths occurring. In addition, other textured breasts implants not made by Allergan were also in the FDA’s statement, including implants made by Mentor and other unknown manufacturers.
At this time, the FDA only requires removal of recalled textured breast implants if you have symptoms associated with BIA-ALCL, which include swelling of the breast or a lump under the skin.


![[UPDATED: VA Senate Dems Pass $15/Hour Minimum Wage Bill] VA House Democrats Pass Top Priority, Paid Sick Leave](https://bluevirginia.us/wp-content/uploads/2026/02/housedemspaidsick.jpg)