With COVID-19 vaccines likely to start rolling out in December, the Virginia government is gearing up to prioritize and facilitate vaccine distribution. For a good overview of what things are looking like at the moment, see below for video of a presentation earlier today by Virginia’s State Epidemiologist Lilian Peake. Here are a few key points:
- As Peake explained, even as the vaccine begins to roll out – possibly as early as mid-December for the Pfizer vaccine – it’s going to take a while, and we’re going to need to keep social distancing, wearing masks, etc.
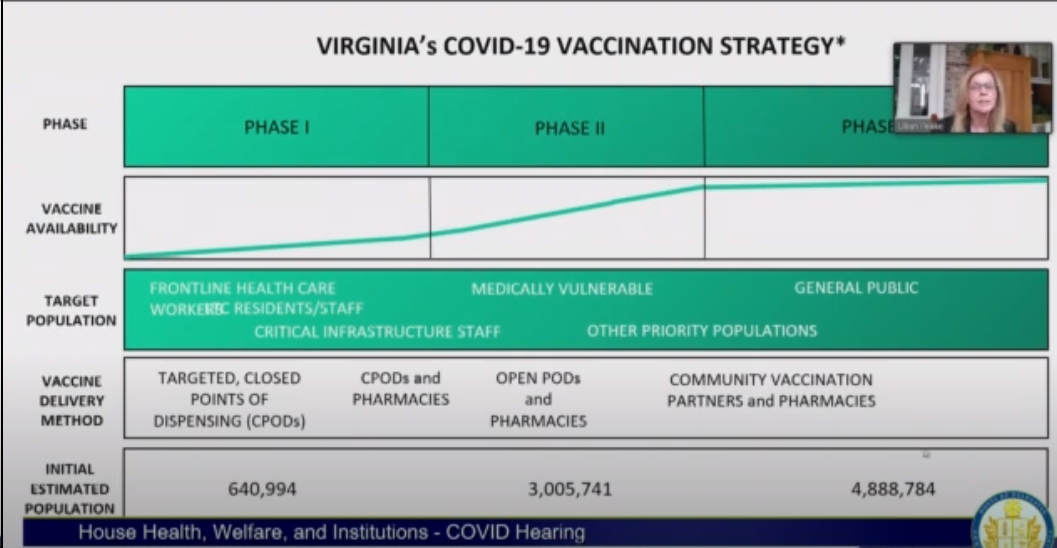
- The vaccine rollout will be in phases: 1) frontline healthcare workers, long-term-care facility residents/staff; 2) the medically vulnerable followed by “other priority populations”; 3) the general population
- Demand for the vaccine is expected to be high.
- The Pfizer vaccine needs to be stored in ultra-cold conditions, so there will be 16 facilities/systems with ultra-cold freezer capabilities where the Pfizer vaccine can be pre-positioned.
- Both the Pfizer and Moderna vaccine require two doses – Pfizer 21 days apart, Moderna 28 days apart. Also note that the Moderna vaccine only has to be frozen normally, not at ultra-cold temperatures. Basically, the vaccines are very similar, and currently there’s no preference for one over the other.
- There will be important partnerships with hospitals, long-term care facilities, medical providers and pharmacies, etc.
- Currently, the are not plans to vaccinate school kids, as the vaccines haven’t been tested on children. So, for the time being, the vaccine will be available to adults only.




![Thursday News: “World leaders now enter the White House at their own risk”; “2 Israeli Embassy staff shot and killed in front of Capital Jewish Museum in DC”; GOP Moves to Pass “Monstrous” Bill Which “steal[s] from the poor and give[s] to the rich”](https://bluevirginia.us/wp-content/uploads/2025/05/montage0522-238x178.jpg)





![Saturday News: “Trump’s latest tariff TACO probably won’t make your life more affordable”; “The Epstein Email Cache: 2,300 Messages, Many of Which Mention Trump”; “[MTG] questions if Trump is still the ‘America First’ president”; “Jim Ryan tells all: ‘What did the Governor know, when did he know it?’”](https://bluevirginia.us/wp-content/uploads/2025/11/montage1115-100x75.jpg)
